By Jim Montague, Executive Editor
Not many car dealers will drive you home. Not many oven manufacturers will bake cookies for you. And not many mattress companies will tuck you in and check for monsters under the bed.
However, a lot of pharmaceutical and biotech manufacturers need some extra TLC these days so they can begin changing decades-old production practices and adopt process analytical technology (PAT) methods. Consequently, some of their machine builders and equipment suppliers are lending a hand.
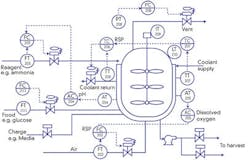
For example, Broadley-James in Irvine, Calif., recently spent a year and more than $1 million to model and start running batches of donated CHO mammalian cell cultures this past January. The bioreactor builder is now producing antibodies in six of its 7-liter, benchtop bioreactor systems. Its conducting this gutsy beta test to prove to potential users that it can accomplish on-line prediction of quality and economic parameters, show the value of high-fidelity process models for testing alternate control strategies and evaluate different means of online fault detection and identification (See Figure 1).
Broadley-James BioNet uses a benchtop version of Emerson Process Managements DeltaV controllers, HMIs and software to bring industrial control capabilities to its bioreactor system, and uses multi-test BioProfile Flex bioanalyzers from Nova Biomedical Corp., to examine 15 parameters from a single 1-ml sample every four hours.
Running Reactors
A technician at Broadley-James checks cell cultures growing in 7-liter bioreactors sampled every four hours by Nova Biomedicals bioanalyzer and monitored by Emerson Process Managements DeltaV.
The beta test also is using single-use bioreactors donated by Hyclone. Its 100-liter bioreactor will be used to prove scale-up of Emersons mammalian cell model, and results are expected by Interphex 2009 next March in New York.
We previously had little information about what was going on in bioreactors. We usually took hand samples every 24 hours and had a pH probe and dissolved oxygen (DO) probe, but we didnt know much about the quantity and quality of cells. If we had this data, we could introduce more control and make more and better quality product, says Trish Benton, Broadley-James life-sciences consultant. Incorporating DeltaV mass-flow controllers and digital I/O allows us to control several variables at once, makes the vessels all perform the same and gives us a nice, precise digital sequence. Having a well-characterized bioreactor system also means we know better what needs to go into the next, larger batches when we scale up.
Catching Up to History
Its lucky some suppliers are willing to give their pharmaceutical users so much assistance because few seem able to update their manufacturing practices on their own. Despite a flurry of interest and activity when the U.S. Food and Drug Administrations (FDA) PAT guidelines were released in 2004, many of the subsequent conferences, publications and attention surrounding them have died down, and observers report that inertia has once again tightened its grip. This paralysis seems driven by fears that any process change may trigger revalidation requirements; the fact that many drug patents are due to run out by 2010-11, increasing layoffs recently; and profit margins that still have been large enough to stifle most production efficiency efforts. As a result, there still dont seem to be more than half-dozen drugmakers with genuine PAT projects underway.
There are competing forces affecting PATs implementation, says Gawayne Mahboubian-Jones, product development manager at Optimal Industrial Automation, a U.K.-based PAT system integrator. The positive forces are the need to reduce the gross inefficiency of manufacturing in the pharmaceutical industry and the need to improve product quality by improving process capability and removing the need for end-of-line testing and replacing it with a better method. However, negative forces resisting this change include pharmas enormously conservative nature, its unwillingness to learn from outside and the ambivalent response of the FDA and regulators worldwide. Pharma believes that its special, and has to develop all its own solutions to problems.
The FDAs PAT guidance was the first real step towards pharma adopting methods pioneered in other industries about 20-30 years earlier. That guidance was driven by the early recognition that the industry was heading into a financial dead-end because of higher drug development costs and fewer new drugs. The industry as a whole still doesnt really understand what the FDA PAT group understood five years ago.
Process-Based Background
Joe Alford, who recently retired from Eli Lilly, Indianapolis, Ind., reports that many people think of PAT as only three or four years old because of the FDAs guidelines, but that his former company and others have been practicing its methods for 20 years as online process mass spectrometry (PMS) and online, high-performance liquid chromatography (HPLC). These have been tried and true technologies for a long time, says Alford. PAT uses them for online and near-real-time testing to monitor process quality parameters and get pre-approval of products. So, instead of waiting hours for test results before harvesting from a bioreactor, PMS now gives enough data so users dont have to wait. Because the FDA also was seen as part of the problem, its resulting PAT philosophy is that users should do what makes the most sense in their applications and then justify those decisions later.
In fact, the FDAs 2002 current Good Manufacturing Practices (cGMPs), on which its PAT guidance and quality-by-design (QbD) recommendations are based, reportedly give drugmakers more leeway to make process changes without traditional validation if they make those changes within a pre-defined design space, according to Brian Stephens, ABBs PAT coordinator for North America. He adds that research indicates that using PAT can reduce cycle times from 25 days to 12 days on average.
The heart PAT is understanding your process and then using that understanding to improve manufacturing operations, adds Bart Reiter, GE Fanucs life-sciences global industry manager. However, especially in pharmaceutical firms, this can require changes in organizational thinking, behavior and funding allocations. Many pharma companies have been manufacturing the same way for 100 years, so a sea change is needed to shift their focus from just making product and onto manufacturing. This is not a technical problemits a cultural problem. For example, one of our service technicians was at a pharmaceutical operation that was still using a ladle in its sugar-coating pans, and I know a guy who reported seeing an operator using a canoe paddle and a stopwatch in a blending operation.
Control to the Rescue
To improve their process knowledge and understanding, some pharmaceutical firms are forming PAT teams and enlisting process control engineers and suppliers to help update their primary and secondary processes from offline sample testing to online or at-line principle component analysis (PCA) of multivariable processes and projection of latent structures (PLS) to begin to accurately predict that a given batch will meet its quality parameters.
Instead of waiting for end-product test results, the FDAs PAT guidelines say manufacturers can release product before testing if they can prove they have enough knowledge of their entire process and the multivariate analysis tools to accurately predict that theyll meet required product quality parameters, says Terry Blevins, Emersons principle process management technologist. For example, Emersons DeltaV Insight software identifies process dynamics with each loop and at each point of a batch, so users can diagnose, identify gains and make process changes as a batch progresses. He adds that Emerson recently helped Baxter Healthcare avoid $6 million in capital revalidation costs by implementing multi-loop process control to the distilling process at a new anesthetic production facility.
Unfortunately, these tasks arent made any easier by the fact that batch systems must deal with numerous different operating conditions during their cycles (Figure 2). For instance, feed-flow, oxygen and/or reagent demands can vary widely during a typical bioreactor cycle, which can make it hard for sensors and controls to adhere to parameters and maintain product quality, so PAT projects often need to implement continuous performance monitoring and control functions. Despite the upfront development and configuration costs, PAT can help users save time and improve quality by moving from manual feeding that can shock batches and increase variability to continuous feed that can improve consistency.
Juggling Operations
Typical bioreactor and other batch applications must handle numerous operating conditions, and so they need ongoing performance monitoring of measurement and control to reduce variability and maintain product quality.
The ability to sample and test every four hours, which Broadley-James bioreactor can do with Novas analyzer, expands our knowledge and allows more impact on the process, adds Blevins. However, these methods need to be designed into processes at the product development stage.
Telescoping Down, Hurrying Up
Likewise, New York City-based Pfizers plant in Ireland recently installed near infrared (NIR) spectrometers to monitor moisture closer to real time when drying its Lipitor product. Drying the pills used to take two days, and then samples were analyzed offline. However, after jointly developing and installing ABBs FTPA 2000 NIR analyzers and software, Pfizer discovered that it was able to dry the pills in one day, and saved about $10 million in one year, says Thomas Buijs, ABBs produce line supervisor for AAS and remote sensing.
Traditional pharmaceutical manufacturing wastes huge amounts of time waiting for lab results that come back too late to optimize its processes, says Buijs. The key factor is being able to measure closer to real time, which allows better optimization and reduces cycle time.
Buijs adds that ABB also helped Novartis, based in Basel, Switzerland, add a small NIR device and battery-powered WiFi equipment to a three-powder blending application in a V-shaped arrangement of two drums to make sure the powders were blended homogeneously. Using NIR meant that waiting for lab results was no longer needed in this application.
Sound Advice, Growing Interest
There are articles and training courses on PAT, but the change is also a mindset change on the factory floor. As long as pharmaceutical manufacturers think of quality in traditional terms, theyll continue to fail to understand the need for PAT or how to apply it, explains Mahboubian-Jones Pharma has a huge amount to learn, but the biggest lesson is that its not unique, that others have similar problems and have developed solutions which make an excellent starting point. One way is to bring in expertise from outside the industry, which is very rare in pharmaceuticals.
One of my mantras when consulting to the pharma industry is, Go out and learn what others have done with the same processes. Food and beverage uses many of the same processes as pharma OSD, while fine chemicals is strongly parallel to pharma API, and the brewing industry would be a very useful lesson for pharma biotech. So start small, look for low-hanging fruit, set aggressive time scales, be prepared get help from external experts, look outside the pharma industry and work hard to ensure that you stay close to a corporate sponsor.
Despite their reflexive resistance, Benton adds that some folks from the major pharmaceutical firms want to learn about PAT and use Broadley-James bioreactor system. Were seeing a lot of interest from the automation guys in the pharma plants, says Benton. However, many of the scientists dont quite grasp it yet, and so theyre still skeptical.
Because its beta test is still running, Benton adds that final results arent available yet. There were also some challenges with getting six sensors installed on the small, 7-liter vessels, which threatened to change the tests characteristics. However, the expected efficiencies and benefits generated by Broadley, Emerson and Novas partnership likely will be groundbreaking. Were looking at substantially reducing the usual 18 months spent on process development, getting more and better quality data that can be used for all ranges of scales, and securing accurate predictions of actual performance if we change the control on a particular variable.
Onto the Plant Floor
Though some drugmakers have PAT in their R&D facilities, moving it into actual processes is another ball game entirely. Pedro Hernandez, Ph.D., QbD and PAT leader at Wyeths (www.wyeth.com) Pharmaceutical Development Center in Puerto Rico and New York, explains that his company began this migration three years ago by acknowledging at all levels that Wyeth needed QbD and real-time monitoring.
We needed and used champions and end users in our organization who committed to saying it was OK to make time for PAT, says Hernandez. Then we drafted feasibility plans, brought in IT, developed new applications, conducted monitoring and statistical analyses, implemented new processes, trained staff, presented internal case studies, and moved toward continuous improvement. The case studies showed people that investing in PAT was worthwhile because it will give us better knowledge and control of our unit processes. You could see the lightbulbs go off in their heads. It's about culture and philosophy, as well as tools and technology. It's a commitment and recognition that QbD, PAT and achieving continuous quality assurance is everybody's responsibility.
As a result, Hernandez adds that Wyeth presently uses PAT to monitor several unit processes like blending, milling, drying and compression across multiple products.
Jim Montague is Controls Executive Editor.
Piloting a PAT ProjectIts not easy to get a process analytical technology (PAT) model and project up and running, but there are a few basic steps that pharmaceutical manufacturers or other process users can take to make sure they implement the most useful solution for their application, according to several veteran phamaceutical manufacturers, process control engineers, system integrators and equipment suppliers.
|

Leaders relevant to this article: