Gregory McMillan is a Control columnist, blogger and a member of the Process Automation Hall of Fame. Read McMillan's Control Talk Blog.
Check out ControlGlobal.com on Google+
Great Expectations and Practical Limitations
The pH electrode offers by far the greatest sensitivity and rangeability of any industrial measurement. A pH measurement with a 0-14 pH scale can cover 14 orders of magnitude change in hydrogen ion activity (hydrogen ion concentration for dilute solutions), and can respond at the popular 7 pH setpoint to changes in hydrogen ion concentration in the eighth digit after the decimal point. But, to achieve their full potential, challenges need to be addressed. Except for unusually harsh streams in terms of high temperatures, low water content or chemical attack of glass, suppliers say most of the reported problems are with the reference electrode.
Measurement Electrode
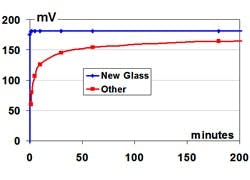
The measurement electrode depends upon the outer glass surface layer exposed to the process being clean, hydrated, with the same activity as the inside glass surface in contact with the internal 7 pH buffer solution. The activity of the glass surface depends upon the concentration of key alkali ions and the number of active sites for the hydrogen ion exchange with water molecules in the glass surface. The millivolt potential develops from an exchange that can be visualized as a jump of the hydrogen ion between a hydronium ion in the aqueous solution and the hydrated glass surface. Abrasion, aging and dehydration of the glass surface can cause a loss of active sites that results, first, in a slowing of the pH response and, finally, as a decrease in the efficiency (span) of the pH measurement electrode. The glass resistance can become too high, resulting in noise. A slight imperceptible coating can prevent the jump of the hydrogen ion, resulting in a dramatic slowing of the glass response. Just a one-millimeter coating can cause the 86% response time of the glass electrode to increase from seconds to minutes. A loss of active sites can cause the response time to increase to hours.
Reference Electrode
The reference electrode must provide electrical continuity from the internal electrode (e.g., silver-silver chloride) through the reference electrolyte fill (e.g., silver chloride), reference junction and process fluid to the measurement electrode. To accomplish this continuity, there is either a very tiny open junction (aperture) or a porous liquid junction to allow ions in the reference internal electrolyte fill to migrate into contact with the process fluid. Unfortunately, process ions can migrate through the liquid junction pores or opening, clogging the junction and contaminating (poisoning) the internals. Additional liquid junctions are added internally to provide additional barriers to prolong the time until the contamination by process ions reaches the inner sanctum of the silver-silver chloride electrode. Also, the internal fill is changed from a liquid to a gel to slow down migration of process ions.
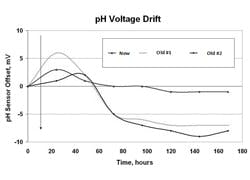
A porous reference such as Teflon provides the ultimate barrier for slowing down poisoning. The electrolyte and internal electrode are changed when the process fluid excessively interacts with the internal electrode or fill. For example, the fill and internal electrode are changed when the process has cyanide because cyanide ions cause precipitation of the silver and attack the internal electrode. The precipitate plugs the liquid junction. A plugging or a coating of the junction slows down ion migration and increases the potential at the junction, causing an increasing offset (drift) in the measurement. Concentrations of reference electrolyte ions build at the reference junction until an equilibrium potential is reached, opposing further migration. Similarly, process ions accumulate at the liquid junction until an equilibrium potential is reached. The potential developed is called a liquid junction potential. For process streams with a large concentration of ions (high ionic strength) the liquid junction potential can result in an offset as large as 50 millivolts. The equilibration rate increases and size of the junction potential decreases as the area and porosity of the liquid junction decreases. Thus, there is a tradeoff between plugging rate and equilibrium rate. Solid reference electrodes greatly reduce plugging and essentially eliminate positioning. However, reference equilibration might take days in a process with high ionic strength. Buffer calibration may not be able improve the accuracy beyond 0.1 pH because repeatable readings may only be repeatable to 0.1 as electrodes are moved from one buffer to another.