To streamline this traditionally difficult process, GlaxoSmithKline's (GSK) plant in Cork, Ireland, recently replaced its legacy, mostly manual calibration method with a combined software solution, including Asset Management Suite (AMS) DM and CMX Professional. The new system has been up and running at GSK Cork for about one year, though its calibration application usually runs in manual rather than automatic.
"We used to have a mostly paper-based calibration process. Forms were filled in by the technicians, and then data was entered manually by the administrators. It was very static system," says Don Brady, GSK Cork's automation engineer. "So, in the first quarter of 2009, we launched our Primary Supply Agile Engineering team, and they confirmed our calibration cost problem, and assigned us to identify ways to reduce these expenses."
GSK is a research-based pharmaceutical firm with about a 5% share of the world's pharmaceutical market. It employs 96,500 people in more than 100 nations, including 13,000 in R&D. Its prescription medicines treat infections, depression, skin conditions, asthma, heart and circulatory disease, cancer and other conditions, and its consumer brands include Alli, Ribena, Horlicks, Lucozade, Aquafresh, Sensodyne, Panadol and Tums.
Linking Software Speeds Up Data Exchange
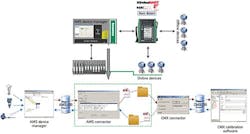
To assist GSK Cork and their other pharmaceutical end users, Emerson Process Management (www.emersonprocess.com) and Beamex (www.beamex.com) recently developed an innovative method for linking Emerson's AMS Device Manager (DM) software with Beamex's MC5 portable calibrators and CMX Professional calibration management software. Basically, this technical partnership allows calibration data to be collected by AMS DM; transferred to MC5 and CMX for analysis, verification and sign off; and then sent back to AMS for documentation and certification. This streamlined process can save users hundreds of hours compared to traditional calibration and validation procedures. This data transfer also is aided by Beamex's CMX Connection software (Figure 1).
Figure 1: Calibration data at GSK Cork collected by AMS DM software; transferred to portable MC5 calibrators and CMX software for analysis, verification and sign off; and then sent back to AMS for documentation and certification. This streamlined process can save users hundreds of hours compared to traditional calibration and validation procedures.
"Emerson recognized that our existing DM tools and strategies might not be enough for users in the more regulated process industries," says Richard Barnes, Emerson's plant asset management and asset optimization consultant. "As a result, we integrated AMS DM and CMX, so they can exchange information with help from CMX Connection and greatly reduce calibration times." Brady and Barnes presented "Calibraton Excellence" and a separate live demonstration of the new AMS DM and CMX tool last October at the 2011 Emerson Global Users Exchange in Nashville, Tenn.
Because of its cooperation with Emerson and Beamex, GSK Cork's calibration improvement project focused on three areas:
- Instrument diagnostics—including shifting calibrations of non-critical instruments from "on schedule" to "on demand," and relying on smarter diagnostics from instruments;
- Paperless calibration—to help shorten calibration time and eliminate non-calibration support functions, such as document management, manual approvals and correction of transcription errors;
- Calibration analysis—to examine historical data and help recommend interval extensions between caibrations, so long as existing data shows that previous calibrations met stringent corporate policy.
"We're trying to achieve calibration efficiencies by using condition monitoring to reduce our total number of calibrations and then performing the remaining calibrations in a timely and efficient manner," explains Brady. "This means synchronizing instrument data between the calibration management system and the asset management system; using documenting calibrators; optimizing scheduling of planned periodic calibrations; and removing paper from the calibration process."
Beady added that GSK Cork is an highly automated manufacturing facility and serves as the primary production site for several of GSK's best-selling products. The plant employs more than 300 people and contains about 4000 instruments, including mostly HART and Foundation fieldbus devices, which were installed over the last 12 years. The vast majority of these instruments are connected to one of the plant's six Delta V systems, which employ classic I/O, remote I/O or Foundation fieldbus I/O.
"Besides being required to have CFR 21 Part 11-compliant signatures for data entry and approvals, we also had to monitor 1100 smart-instrument diagnostics, develop an audit trail for 1100 changes to that smart instrumentation, and electronically document our smart-instrument configurations," adds Brady. "We also had full participation from staffers, who did training and learned how beneficial the new calibration system was going to be. Our craft supervisors and technicians were included in the project from day one, so there were no operational surprises by the time we reached the go-live date."
Calibration, Asset Integration Yields Big Benefits
Brady reports that GSK Cork's calibration improvement project, including using the combined AMS DM and CMX, helped the plant to:
- Reduce the steps in its overall calibration process from 17 steps to 10 steps;
- Remove two steps that each posed risks of human data-entry errors;
- Conduct Emerson and Beamex consulting workshops to review existing processes and optimize and refine the new processes to use improved technologies;
- Use the new system's flexibility to manage workflow in addition to carrying out its primary function of managing calibrations.
For instance, its integrated asset management and calibration solution has allowed GSK Cork's staff to obtain history reports and identify adverse trends so they can reduce calibration frequency without hindering production or quality. In fact, CMX also helped find more than 100 hours of savings in its first three months of operation. Also, one-third of lower-criticality instruments, which aren't directly related to quality or regulatory requirements, were moved from scheduled calibration to calibration on demand. This means an instrument's own self-diagnostics can trigger a work order for calibration. GSK Cork reports this method has saved about 300 hours per year.
"We've also eliminated 21,000 sheets of paper per year," adds Brady. "And we streamlined our end-to-end workflow, which reduced our calibration time by about 15 minutes per calibration. We've also eliminated calculation errors and rework because pass/fail calculations are performed in real time out in the plant. In addition, we reduced scheduled calibrations by 8%, which was part of the data migration from legacy system. We're also generating email alerts when a calibration failure happens, but now only calibration failures need to be reviewed and approved. Finally, we've been able to extend some intervals between calibrations, which are recommended by the devices and system in the form of email alerts. So far, we removed 234 calibrations from our schedule during first 12 months of analysis, and this should peak during the third year of operation."