HEY, HEY! The control system train has pulled into the pharmaceutical station! All aboard! Though PAT most directly applies to pharmaceuticals, its effects will be felt in other industries regulated by the U.S. Food and Drug Administration (FDA), such as food and beverage, as the FDA tightens its regulatory grip to include all ingestible products.
PATs benefits included reduced production cycle times, improved manufacturing efficiency, reduced rejects, and increased production uptime. PAT can also speed time to market for new products, improve operator safety, and improve relationships with regulatory agencies.
Encouraged by the FDA, the pharmaceutical industry is seeking to accelerate its manufacturing innovations. While it continues to spend on research and marketing, the pharmaceutical industry lags behind other automated process industries in manufacturing productivity.
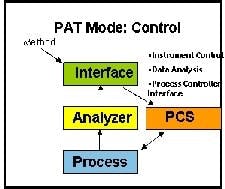
In the second paradigm, Control the CQA are monitored and controlled to limit or manage process variance within the design space and to ensure end product quality. The PAT software usually will be interfaced to Process Control Systems (PCS) that perform this control. (Click chart to enlarge)
To improve productivity, there is growing enthusiasm in pharmaceuticals for PAT, an FDA initiative to improve manufacturing efficiency and product quality, while also harmonizing regulatory expectations. PAT provides a framework for designing, analyzing, and controlling manufacturing. The PAT initiative focuses on the principles of building quality into products and processes, as well as continuous process improvement.
New century, new hope
Historically, innovation in pharmaceutical manufacturing was largely constrained by regulatory uncertainty. With the subsequent launch of its GMPs for 21st Century initiative, the FDA began calling for innovative approaches for process development, manufacturing, and quality assurance (QA). This was a paradigm shift that required quality to be designed in and not tested into products. Designing quality into products requires a comprehensive understanding of the process, including the impact of product components on process variability, along with mechanisms to manage the process.
Continuous improvement is a critical element in a sound quality system. The FDA expects pharmaceutical manufacturers to implement continuous improvement through the PAT framework. In addition to continuous improvement, the PAT framework also encompasses risk assessment, knowledge management, and on-line analysis.
Though the FDA published guidance on Pharmaceutical cGMPs for the 21st century to enable innovation and continuous improvement, specific GMP regulations have not yet changed. Despite this delay, the FDA is providing science and risk-based guidance related to GMPs.
Consequently, PAT will help pharmaceutical manufacturers design, monitor, control, and predict process performance. Many of these functions are now implemented separately, but PAT promotes an integrated environment that combines modeling tools for design/analysis, process analyzers, and process control/optimization. Knowledge of all these functions is required to efficiently apply these technological innovations to pharmaceutical manufacturing.
So, how do we in the pharmaceutical and other regulated industries implement PAT successfully? How do we hop on the control system train?
We must first bridge several gaps in existing infrastructure and control system architectures. This article presents a strategic framework for managing this transition effectively. We also identify a new standard PAT software platform that will supplement and improve existing control system architectures.
PAT monitors, controls, optimizes
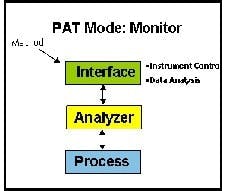
The most basic implementation of PAT is process monitoring. This involves monitoring critical-to-quality (CQAs) variables to build process knowledge, establish process variance, and set the design space in which the product is robust (See Figure 1 above).
The next level up in PAT implementation adds control to the basic process monitoring functions. CQAs are now monitored and controlled to limit or manage process variance in the design space to ensure product quality. PAT software must be interfaced (See Figure 2 below) to a process control system (PCS) that performs real-time control.
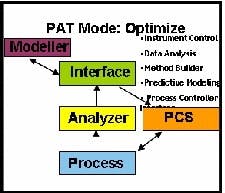
The highest level PAT implementation adds optimization to the monitoring and control functions (See Figure 3 below). Optimization reduces process variance and optimizes process capability by running controlled Design of Experiments on the CQAs.
Optimization reduces process variance and optimizes process capability by running controlled Design of Experiments on the CQAs.
(Click chart to enlarge)
CQAs can be predicted based on history and the performance of the process by using a set of relationships that have been created with mathematical models. These predictions can be made available to the PCS for timely control.
PAT standardization fights complexity
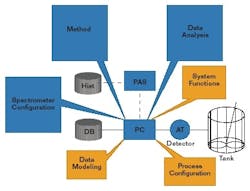
A typical PAT System incorporating monitoring, control and optimization (See Figure 4 below) needs to acquire data, control the process, run prediction models, and display results. This implementation is easy to handle with one instrument, but grows exponentially more complex in typical applications with hundreds or even thousands of sensors and analyzers. Each instrument has its own process and instrument configuration, data modeling, method builder, and data analysis capability.
- Reducing validation costs for PAT devices
- Minimizing deployment time for PAT technology
- Improving PAT system robustness
- Reducing custom interface code
- Providing flexibility to repurpose PAT devices based on evolving needs; and
- Improving scalability and extensibility
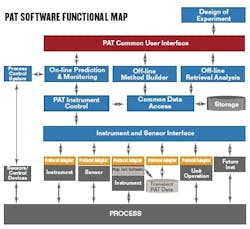
A functional map implementing a PAT software standard (See Figure 5 below) shows the Instrument and Sensor Interface has a common interface for all process sensors, analyzers, and instruments. The control, operation, analysis, retrieval and prediction functions all use the PAT Common User Interface. During on-line operation, the user interface provides access to select appropriate methods, and generates results from predictions versus time. The interface also reports status of prediction alarms and instrument alarms with appropriate alarm management functions. In off-line mode, the interface provides access to the method builder, PCS configuration, and Design of Experiments setup.The user interface manages the PAT hardware configuration, and performs analyzer calibration functions. Any data retrieval from data storage takes place through the interface.FIGURE 5:
To acquire, process, and execute real-time predictions, a PAT method needs to be created. This is usually created off-line and executed on-line in real-time. The method builder controls active modules, and adjusts integration time, exposure time, and signal gain or amplification. It also handles summations or averages of each data acquisition to generate a spectrum. Once the analytical information is generated, the method acts on this information via various mathematical pre-treatments and prediction engines. The traditional approach uses quantitative models. Single or multivariate models generate calibration models, real-time analytical data, and predictions. However, the need to generate these values is driven more by perception than necessity. This is changing and qualitative and trend models are now widespread, inspiring the term process signature.All data should be stored in a way thats easy to access for analysis. Raw data files should be converted to a standard format such as Analytical Information Mark-Up (AniML, ASTM E13.15).Events and context-based process data from other sources also should be available. Data storage must handle structured and unstructured information. Implementations can include existing data storage mechanisms, but these may need to be scaled and extended to new types of information. This data storage can be integral to the PAT software or it can be a part of a common data repository.How do we get there from here?
Creating a PAT software standard will require committed suppliers and end-users working together. We anticipate that common functions will be identified first, and that products will then emerge. We also propose that end-users take advantage of standard PAT software when its available. The first step is to standardize complex measurements. Spectroscopy methods are routinely adapted for PAT. Examples are near infra-red (NIR), Raman, UV-visible, fluorescence, and acoustic. Measurement technologies suitable for pharmaceutical needs must be identified. This will help suppliers meet implementation challenges and simplify deployment.Presently, we spend a lot of time specifying a measurement for a process application. We would like to see standards created that will reduce required time, and make time commitments more predictable. In the most advanced implementations, PAT systems are used for monitoring, control, and prediction. In these cases, data needs to be exchanged with systems that perform analysis, control, decision making, and reporting. A good first step for data exchange in advanced implementations is to create standard ways of trading data across existing systems.Control system architecture impacts
Weve heard about several efforts to build master plans for deploying innovative measurement technologies. Most are done in isolation without considering effects on control system architecture, existing applications and infrastructure needs. However, any master planning effort has to consider these impacts. The master planning effort must align strategies and derive specific objectives for infrastructure, applications, integration, and PAT systems. To do this effectively, all functions must be mapped to domains. New functions, such as process optimization and process improvement, should be considered. For each of these functions, impacts on level and approach to automation must be considered. This master planning exercise will help end users understand their business readiness to embrace continuous improvement outlined in the PAT framework.We all have to deal with existing systems and infrastructure, even when creating PAT software standards. Well have to exchange data with existing systems for process control, enterprise resource planning, laboratory information management, and manufacturing execution. A unified schema is critical to facilitate data exchange among these platforms. Several organizations have developed schemas focusing on standard data related to functions of relevance. These include Analytical information Markup Language (AniML), Batch Manufacturing Language (BatchML), and Business to Manufacturing Markup Language (B2MML). While these schemas have been around for awhile, corporate schemas must be developed to use them effectively. Schema validation services for the entire pharmaceutical industry also will be required to support new PAT software standards.To facilitate pharmaceutical manufacturing process improvement and innovation, application architectures bow geared to corrective action must instead focus on continuous improvement. Current application architecture domain models must evolve to address continuous improvement.
|
REASONS FOR IMPLEMENTING PAT |
| About the Authors |

Leaders relevant to this article: