Key Highlights
- Proper sensor design, installation and maintenance are vital for preserving the integrity of pH measurements, especially given the sensitivity of glass electrodes and reference junctions.
- Understanding the electrochemical principles, including proton transfer and activity coefficients, helps in selecting suitable electrodes and interpreting measurement data accurately.
Greg: Data integrity is greatly affected by process sensor design, installation and maintenance. This is particularly true for pH measurements that have an incredible sensitivity and rangeability many orders of magnitude greater than any other composition measurement. The logarithmic relationship between pH and hydrogen-ion activity offers the ability to measure hydrogen-ion concentration from 1 to 10-14 over the 0 to 14 pH scale range. In fact, pH measurements below 0 and above 14 are possible, extending the rangeability beyond 14 orders of magnitude.
In this column, I engage in a conversation with “pH Greg” to learn about the problems and solutions for enabling electrodes to achieve their extraordinary capability.
pH Greg: The pH sensor measures hydrogen-ion activity that is the effective concentration and s a measure of the hydrogen-ion’s ability to move and combine with other ions. For dilute solutions, the effective and actual concentrations are equal, and the activity coefficient is 1. For solutions with high concentrations of ions, the crowding and presence of other charges reduces the activity coefficient to less than 1. For solutions with less than 90% by weight water or more than 5% by weight salt, the pH becomes a noticeable function of water and salt besides hydrogen-ion concentration. Certain salts, such as NaCl, also affect the millivolt potential developed by the measurement and reference electrodes. The ions from the dissociation of acids and bases also have activity coefficients that affect the charge balance and the pH.
aH = 10-pH (1-1a)
pH = – log (aH) (1-1b)
aH = γ * cH (1-1c)
where:
aH = hydrogen-ion activity (gm-moles per liter)
cH = hydrogen concentration (gm-moles per liter)
γ = activity coefficient (1 for dilute solutions)
pH = negative base 10 power of hydrogen ion activity
Greg: Start us out with a dose of reality.
pH Greg: The incredible sensitivity and rangeability of the pH measurement are based on a series of assumptions, most of which are not sufficiently explained in the literature. Manufacturers do a better job of noting some considerations, especially as they develop new electrode features that address a particular application problem. More than any other sensor, there is a need for pH electrodes to “keep it real.”
The accuracy and speed of response of the pH measurement depend on the condition of a hydrated gel layer on the surface of the glass electrode that is only 10-4 mm thick and the condition of a junction of the reference electrode that provides physical contact between its internal electrolyte and the process solution. The need for the fragile gel layer and the process junction of the reference electrode to be structurally consistent, clean and free from contamination, has profound implications for properly selecting, calibrating and maintaining pH measurements. The fact that pH sensors perform as well as they do despite exposure to a wide variety of chemicals, temperatures and pressures is remarkable and a testimony to the ingenuity of various electrode designs.
Greg: What are some fundamentals?
pH Greg: The sensing of pH is accomplished by having a pH-sensitive glass in contact with the internal fill, a 7-pH buffer and the external solution. The pH-sensitive glass develops Nernst equations by the hydrogen-ion (proton) exchange between hydronium ions in aqueous solutions and in the hydrated gel layer of the glass. The migration of hydronium ions through the boundary layer that surrounds the surface takes several seconds even under the best of conditions, but once the hydronium ions are in very close proximity to the surface, protons jump from the hydronium ions and become associated with water molecules on the surface.
There is no migration of hydronium ions intact into the surface but rather a proton transfer that occurs for aqueous and nonaqueous solutions. As protons move into exterior surface from a greater dissociation of hydrogen ions, they move out of inside glass surface. Lithium and sodium ions in the dry interior of the glass provide electrical continuity between the internal and external hydrated gel layers. The potential developed is proportional to the difference in logarithms of the activity of hydronium ions in the solution and in the gel layer on each side of the glass membrane.
Water molecules in the glass surface are essential for the proton transfer to occur. Nonhygroscopic glasses such as Pyrex and quartz show no pH response. Glasses that can absorb water but have lost their gel layer from contact with nonaqueous and highly acidic or basic solutions or from long-term exposure to air will lose their pH response. It takes about two hours of immersion in water to replenish a completely dehydrated gel layer.
Instead of a sharp transition between the gel and dry layers, there is a continuous decrease in the number of hydronium ions and an increase in the number of lithium or sodium ions from the surface to the interior of the glass. As the electrode ages, the outside surface is stripped away, and the gel layer penetrates deeper into the glass. Eventually, there is a complete breakdown of the silicon oxygen network that forms the glass matrix. For ideal conditions (25 °C and zero ionic strength solutions between 6 and 8 pH), a satisfactory glass response should last two years. Most of the variation in life expectancy for these conditions is associated with the manufacturing tolerances, with the greatest variability shown for manual glassblowing and assembly. Chemical attacks from strong alkaline and acidic solutions, high temperatures, and nonaqueous solutions greatly accelerate corroding and depleting the gel layer.
Greg: What is pH accuracy?
pH Greg: From an accuracy standpoint, the primary concern is drift or bias developed over time. When pH electrodes are calibrated, the calibration line is characterized by a slope, noted as an efficiency, and asymmetrical potential, noted as an offset. The asymmetry is defined as the potential difference across the membrane when the external solution is at pH 7 and 25 °C.
While the slope represents the pH responsiveness of the glass, the asymmetry represents all the remaining deviations from ideality in both the measurement and reference electrodes. The drift in asymmetrical potential for a standard set of electrodes sitting in a buffer solution under ideal conditions in an environmentally controlled laboratory is about 0.002 pH units per day.
Tests for drift should be done with acidic buffers because the actual pH of basic buffers exposed to air would continually drop from absorbing carbon dioxide. In practice, it is difficult to sort out the electrode drift from changes in the solution pH.
Regarding precision, the short-term repeatability for the constant operating and equipment conditions of a standard set of electrodes in mild process solutions is about +0.01 pH. Reproducibility (different conditions or equipment) is usually in the range of 0.03 to 0.04 pH.
Optimistic users and suppliers who provide one electrode per point in the process may believe they have achieved an accuracy of 0.02 pH or better. Results of installations with three electrodes per point in the process show that the error band from mixing noise and reference electrode drift over a month is, at best, 0.02 to 0.1 pH for a flat titration curve and 0.2 to 0.5 pH for a steep titration curve. Tests of 12 different electrodes in a recirculation line of a laboratory tank showed that right after buffer calibration, readings differed by more than 0.5 pH for water and were reduced to about 0.25 pH when buffer solutions were added to the tank. My plant experience has been that after a buffer calibration, three healthy electrodes will differ by 0.2 pH, where an electrode that is higher than the others may be lower within hours. This confirms some of the many advantages of middle signal selection (MSS) in terms of reducing the effect of these fluctuations and providing much more intelligence for calibration and diagnostics.
If all three electrodes continually agree within 0.01 pH for more than a few hours in an industrial application, all three are coated, broken, or still have protective caps.
The actual pH of the solution changes in response to the change in the ion dissociation constant with temperature. Until recently, compensation of only the change in millivolts generated per pH unit, or in other words, electrode temperature compensation, was offered. Microprocessor-based transmitters and receivers now offer solution temperature compensation. However, the relationship depends on the composition and operating conditions of the process stream.
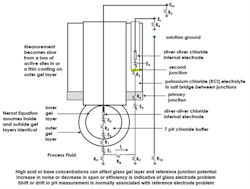
The potential of interest in pH measurement is the difference between the potential developed at the outer and inner glass surfaces of the measurement electrode. All other potential represents an error. Figure shows the physical location of the potential (E1 – E2) and many extraneous potentials for a combination double-junction electrode, which has a reference electrode as a concentric ring around the glass measurement electrode. The combination electrode is popular because it reduces the installation and spare parts requirements. The internal salt bridge between the outer and inner junction of the double junction increases the time it takes for process ions to migrate to the inner chamber where the internal silver–silver chloride electrode is located and therefore increases the equilibrium time when an electrode is put into process.
The effect of these extraneous potentials is additive. Whereas errors from changes in the parameters due to measurement electrode glass result in horizontal shifts of the isopotential point, the extraneous potentials result in a vertical shift of the isopotential point, Usually, there is no attempt to distinguish and compensate for a shift in the isopotential point that is horizontal from changes in the condition of the gel layer versus a shift that is vertical from changes in the path resistance and condition of the reference electrode.
Greg: What are the installed electrode resistances?
pH Greg: Some of the resistances in are large. Fortunately, the input leakage current (Ii) that flows through these resistances and the amplifier input is extremely small (about 1 picoamp or one trillionth of a milliamp). The current flows from the positive measurement electrode terminal to the negative reference electrode terminal, so the sign of the potential drop is negative compared to the measurement electrode’s outer potential and causes the isopotential point to shift down and the pH measurement to go upscale.
The measurement electrode glass bulb resistance is usually the largest resistance. It ranges from about 20-500 Mohms at 25 °C, depending on the type and thickness of the glass. Rugged glasses, which are thicker than normal for greater resistance to breakage and abrasion and for a longer life in terms of time to failure of the silicon oxide matrix from penetration of the gel layer, often have a lower resistance per unit thickness so the increase in total resistance is moderated. The resistance dramatically increases at low temperature and can exceed 1,000 Mohms for the type of glass used that is usually reserved for concentrated acidic solutions at high temperatures. However, advances are being made in glass formulations that increase the range of permissible operating conditions.
A high total electrical resistance from applications with high-resistance glass at low temperatures (<20 °C), with dehydrated glass, or in nonaqueous and pure water streams causes the pH measurement to be sluggish and more susceptible to noise from ground paths, streaming potentials (static electricity), fluid velocity, and electrical interference.
A decrease in the high-impedance shunt resistance (R11) can cause a loss of the electrode slope (efficiency). The degree to which the measured potential is decreased depends on the relative magnitudes of the glass electrode impedance and the shunt impedance. In one case of bad sensors with low shunt resistances, the sensors had low slopes (short-spanned) at room temperature but had adequate slopes for a higher temperature. This was due to the decrease in glass impedance at the higher temperature, which decreased the effect of the high-impedance shunt.
How bad can it be? If you look at the resistance (R11) of the high-impedance shunt as a multiple of the resistance (R1) of the high-impedance glass electrode (R11 = n * R1), you can get an idea of how much the measured electrode efficiency would be lowered [8]:
n efficiency
100 99%
50 98%
20 95%
10 91%
5 83% (bad electrode)
The glass electrode impedance significantly changes with temperature, whereas the shunt resistance does not. As the temperature increases, the glass electrode impedance decreases, what we might call the effective n value due to the shunt resistance increases, and the effect of the shunt decreases. Conversely, as the temperature decreases, the effect of the shunt increases.
The decrease in the shunt resistance (R11) can be accelerated by aging from the exposure of O-rings and seals to a harsh process, which could attack the construction materials and introduce some leakage, as well as from the effects of prolonged heating on the internal potting materials, which could break down over time. The decrease has little to do with the glass electrode itself but rather is related to the integrity of the sensor construction over prolonged periods. It is also likely that at least some high-impedance shunting may be built into the sensor.
Get your subscription to Control's tri-weekly newsletter.
Greg: What is the isopotential point?
pH Greg: The pH where the temperature effect on millivolts generated is zero is known as the isopotential point. For glass electrodes where the voltages associated with the inner chamber of the measurement glass electrode and the reference electrode are the same, the isopotential point is usually 7 pH.
There can be horizontal shifts and vertical shifts for problems with the glass and reference electrode, respectively, of the isopotential point. Contamination of the reference electrode’s inner chamber can shift the isopotential point, which is one of the reasons to use flowing junctions, multiple chambers and solid-state reference electrodes. When glass electrodes are not used, it is critical to adjust the isopotential point.
The isopotential point of the Pfaudler Glasteel material is typically between 1 and 3 pH. The isopotential point of ISFET and metal oxide electrodes depends on the electrode design and, consequently, the supplier.
Greg: What about measuring electrode efficiency?
pH Greg: A loss in efficiency shows up as a decrease in the slope and span of the pH measurement. Abrasion from solids, etching from hydrofluoric acid attack, scratching, loss of Li+ ions, and aging from high-temperature exposure and all other conditions and designs that result in an increase in electrical resistance of the glass also decreases efficiency. Lithium and rare earth ions leave the outer layer when exposed to low and high pH and low water content. These are replenished by lithium and rare earth ions from the interior glass. The gradual loss in efficiency ends up as a rapid drop to zero efficiency when the supply of these ions from the interior glass is depleted.
Although the effect of a shortened span may not be noticeable for operation near 7 pH because the decrease in pH change is small, possibly within normal fluctuations, an increase in the response time often associated with a loss in efficiency is problematic.
Greg: What about electrode response time?
pH Greg: It is critical that the percentage of final responses be noted in any response time statements. Unfortunately, this is rarely the case, and the term final response time is used.
The most representative response time is 86% of the final response (T86) because the plot of pH versus time can flatten out dramatically for the last 10% of its response.
For feedback control, the controller sees enough in T86 response time to correct sufficiently for the problem, especially considering that the accuracy of the measurement is probably less than the last 10% of the response. Because the dead time in the electrode response is usually negligible, the T86 response time corresponds to two-time constants. The T86 response time is used in ISA-75.25.02, Technical Report for Control Valve Response Measurement from Step Inputs, for many of the same reasons.
The general-purpose glass spherical bulb has the fastest T86 response time, about 3 seconds for velocities faster than 2 fps in buffer solution. The spherical and dome bulb and the new high-temperature AccuGlass are almost as fast. Flat glass and rugged glass electrodes can have a response time of 5 to 50 times larger. Aging and fouling can cause a response time to be 100 to 1000 times larger.
Greg: What is the reference electrode equilibration time?
pH Greg: The time for the reference electrode to reach a constant junction potential, known as equilibration time, is usually not stated in the literature or considered in the selection, installation, and calibration of electrodes. It can range from seconds for flowing junction to minutes for liquid junction to hours to days for solid state electrodes.
Greg: How do deal with high and low temperatures?
pH Greg: For high-temperature process streams, the glass type and reference internals must be rated for the time duration of the highest temperature, acid, and base concentrations. The alkalinity error is larger, and the chemical attack is dramatically greater at high temperatures. Life expectancy of the electrode falls off exponentially based on the temperature, acidity, and alkalinity of the process streams.
At low temperatures, the dramatic decrease in glass resistance results in signal noise. Glass electrode resistance doubles for every 7°C decrease, going from about 100 megohms at 28°C to about 1600 megohms at 0°C. The reference junction potential may also be affected. Lower process temperatures lower the activity and the dissociation constants of solvents, acids, and bases, lowering the actual solution pH. Buffers and samples should be at process operating temperatures, which can be challenging for very cold solutions.
Manufacturers generally offer several different glass formulations that are tailored to meet a process requirement. Glasses designed to be more rugged and handle chemical attacks from strong bases and strong acids tend to have higher resistance and consequential accuracy limitations, particularly for wide pH ranges. High measurement electrode resistance can also cause the pH measurement to be sluggish and more susceptible to noise from ground paths, streaming potentials (static electricity), fluid velocity, and electrical interference. Progress has been made in reducing the impedance of glasses so they can be used at lower temperatures.
Glass formulation is a highly proprietary technology with many undisclosed effects, compromises, and consequences as to the ability to handle process conditions. Corrosion strips away the outer gel layer while aging leaches out earth ions, increasing the glass impedance.
Fewer earth ions make pH glass like regular glass. Both types of glass sense pH, but regular glass impedance is too high. More doping with earth ions to decrease impedance per unit thickness makes glass softer and more easily dissolved. Glasses used for thicker glasses in rugged glass electrode design use doping to reduce the total glass impedance with the consequence of being more susceptible to etching. Even with more doping, the extra thickness can make the glass impedance still too high at low temperatures.
A high-temperature glass formulation (AccuGlass) offers significant capability of measurement of higher temperature streams with much greater life expectancy, less drift, and incredibly faster response. The improvements are largely associated with greatly reduced glass aging. There is more than a 100% increase in life expectancy. There are also three orders of magnitude decrease in the 86% response time of new versus general-purpose glass electrodes after exposure to high temperatures. This relatively new high-temperature glass formulation performs as well as a traditional general-purpose pH electrode for less difficult operating (e.g., 25 o C) conditions.
The most viable alternative for prolonging the life expectancy of electrodes in a hot stream is an automatic retractable assembly. As with any electrode assembly, the construction materials must be checked for their resilience to chemicals and temperature. Corrosion increases at higher temperatures, so more attention must be paid to all wetted parts. Solutions can be switched to condition, diagnose, and calibrate the sensor, and the insertion versus the retraction time can be optimized. To avoid thermal shock, the probe should be kept in a relatively hot but neutral solution for the condition. This sample-hold methodology adds an average dead time of about half the retraction time and can only attenuate the peak error of upsets whose time constant is larger than the dead time. Thus, a large back-mixed volume at or upstream of the control point is essential.
Greg: How do you deal with solids?
pH Greg: For solids, the goal is to keep the glass surface and reference junction clean and in good condition. The best inherent method to prevent the start of many coatings is to increase the fluid velocity to 7 fps. However, high velocities are not effective at removing existing coatings. Thus, the electrodes must never be exposed to low velocities. The velocities at the electrode tips of submersion assemblies and flow-through cell holders rarely exceed 1 fps. High velocities are usually achieved by using retractable or insertion assemblies in recirculation lines. Abrasion increases with velocity along with the risk of damage from clumps of material and foreign substances. Therefore, it is important that electrodes be installed downstream of pumps that have suction strainers. Also, velocities higher than 10 fps create more noise and reduce glass life.
Flat surface glass electrodes can provide a sweeping action at a much lower velocity and eliminate the separation of the flow stream and stagnation that occurs on the back side of a glass bulb. Thus, for abrasive, sticky, and viscous streams, flat surface glass may decrease the fouling and stagnation and enable a more representative measurement of changes in the 4 to 10 pH range. The 1 fps velocity in a flow-through holder may be sufficient to keep the glass clean. However, the flat glass formulation has a high resistance requiring a minimization of thickness. Tests show significant vulnerability to cracking, slower response, greater vulnerability to chemical attack, and loss of accuracy outside the neutral region. For these reasons, semispherical or dome glass bulbs with a protective shroud and a higher fluid velocity are preferred and, if necessary, jet washing.
If a spherical or dome glass bulb measurement electrode is used, integral jet washers can be used to provide in-service cleaning. High-velocity nozzles pointed at the bulb are an option for submersion and flow-through assemblies. The pH controller should automatically switch to manual during the jet washing because the signal will be erratic. . If the cleaning solution contaminates the process, automated retractable assemblies can be used to withdraw the electrodes from the process into a chamber where it is flushed, cleaned, calibrated, and conditioned by switching in appropriate streams. The last thing you want in terms of maintenance cost, electrode life, and safety is for technicians to manually clean electrodes. Rubbing and handling electrodes reduces their life expectancy and reproducibility. Brush cleaners attack the outer gel layer and smear sticky stuff onto the bulb, not really cleaning the reference junction.
Greg: What about high pH and alkalinity error?
pH Greg: For high pH streams, the concerns are alkalinity error and chemical attack of the glass. While alkalinity error is often plotted versus pH, it does not have to be associated with high pH because it could be caused by the presence of a high concentration of salt, such as sodium chloride, instead of sodium hydroxide (caustic).
Alkalinity error is caused by alkali ions such as sodium ions penetrating the measurement electrode silicon-oxygen network and displacing the hydrogen ions in the gel layer, which causes a decrease in the hydrogen ion activity of the gel layer relative to the process and a decrease in the measured pH. The high pH end of the millivolt versus pH line bends upward, which results in an indicated pH that is lower than the actual pH. The error is greatest for glasses with alkali ions with an equal or larger radius than that of the alkali ions in the solution. Glasses with small lithium ions are called tight matrix. Alkalinity error is called sodiu- ion error because sodium ions are more prevalent and more of a problem because of the popularity of sodium hydroxide as a reagent and the smaller radius of the sodium ion and, therefore, the greater capability to penetrate the matrix compared to the next most common alkali ion, the potassium ion.
Greg: What are the challenges with nonaqueous solutions?
pH Greg: For nonaqueous solutions where there is a different solvent than water, the issues are bulb dehydration, electrical resistance, electrolyte precipitation, and the relative nature and expanded range of the pH scale.
To minimize the effect of bulb dehydration and the increase in solution electrical resistance, use a large area bulb with a low-resistance glass. Because this type of bulb is more susceptible to breakage, it is important to shield it from direct impingement and reduce the fluid velocity as much as possible without increasing the propensity for coatings. The hydrated gel layer must be replenished periodically, which is best accomplished by an automated retractable sensor. The electrodes should be cleaned with a solvent that will dissolve process solutes; rinsed with a volatile solvent, such as acetone, to remove the cleaning solution; and, finally, soaked in plant water to replenish the gel layer. Any calibration check should be in buffers with the same solvent as the process stream. As with other low-conductivity fluids, there is a heightened sensitivity to fluid velocity and streaming potentials from static charges.
The normal potassium chloride (KCl) saturated in water electrolyte should not be used because KCl will precipitate in a nonaqueous solvent, the water will form a separate phase, and the ions migrating into the junction will have unequal mobility, setting up a large diffusion potential. It is easiest to change the electrolyte in the outer chamber of an internal salt bridge in a double-junction reference electrode. An external reservoir and salt bridge can be used to minimize the frequency of refilling the electrolyte and provide a great level of protection. Common choices for electrolyte solutions for nonaqueous processes are lithium chloride (LiCl) in acetic acid, ethanol, or methanol, and potassium iodide (KI) in acetic acid, acetone, or methanol. Candidate solutions should be tested by soaking the electrodes overnight in the process and measuring the drift.
The pH of a nonaqueous solution can be below 0 pH for an acidic solvent, such as acetic acid, and above 14 pH for a basic solvent, such as an amine. The pH measurements can only be compared to measurements in the same solvent because the dissociation constants of acids, bases, and solvent; the solvent’s dielectric constant; and the ionic mobility and consequential activity coefficients will all be different. The neutral point is not necessarily 7 pH. For example, at 25°C on the water-based scale, the neutral point of methanol is 8.42 pH, and it is 9.55 pH for ethanol. The buffer solvent should be as similar as possible to the process solvent. If a batch process moves from an aqueous to a nonaqueous composition, the readings cannot be compared between phases, but an automatic retractable assembly can enable cleaning, buffer, and soaking solutions that match the sensor calibration and conditioning with the batch phase. If the water content drops below 5%, the solution can be nonaqueous
Greg: What is best for low ionic strength streams?
pH Greg: In low ionic strength streams, such as boiler feed water, demineralized water, and deionized water, the electrical resistance of the solution is high, making the measurement more susceptible to electrical noise and streaming potentials. Low-resistance glass, large spherical bulbs, and an upstream ideally flowing junction reference electrode should be used. The electrode assembly should be shielded from electrical fields and isolated from ground potentials. The solution temperature should be measured. The sample flow rate must be regulated to keep the velocity low and constant.
Greg: What conditions are problematic for reference electrodes?
pH Greg: For cyanides, bromides, iodides, sulfides, or nitrates, the concern is the reactive precipitation of the silver and potassium chloride in the electrolyte, which is observable as a blackening of the junction. The solution is to use an electrolyte in an internal replaceable or an external salt bridge that is compatible with the process, a differential electrode, or a solid-state reference electrode with an immobilized electrolyte or a nonporous junction.
For fluctuating and high pressures, the principal concern is the separation and contamination of the reference electrode. Gel-filled electrodes can develop voids. Liquid-filled electrodes will suck in process fluids when the pressure in the process is greater than in the reference. Solid-state or pressurized reference electrodes with an internal or external salt bridge should be used. Ideally, the differential pressure between the process and the salt bridge should be constant to maintain a constant flow of electrolyte.
For concentrated surfactant solutions, the reference junction potential rapidly shifts, and the surfactants tend to “salt out” in the reference junction when they contact the concentrated electrolyte solutions. Because the surfactants are conductive, neither of these effects correlates with reference junction impedance. The only way to detect these problems is to remove the sensor from the surfactant, rinse it with deionized water, and place it in a buffer solution. Depending on the concentration of the surfactant and the length of process exposure, you may observe an offset in the range of 0.2 to 0.8 pH.
Greg: What are some good installation practices?
pH Greg: The sensor location should provide the most representative, reliable, and fastest measurement. The electrodes should measure the pH of a perfectly mixed combination of feed or influent streams and reagents with minimum maintenance, time delay, and lag.
In general, pH sensor location should be chosen to meet the following objectives:
- Safe and convenient access for calibration and maintenance;
- Permissible process conditions (e.g., temperature and pressure);
- Representative mixture (no short-circuiting of reagent, stagnation, or layering);
- Optimal fluid velocity for minimum coatings and abrasion; and
- Minimum transportation delay.
Greg: What are the advantages of middle signal selection?
pH Greg: Using three electrodes will protect against a single failure of any type including one at setpoint. It can reduce noise and eliminate spikes much more effectively than transmitter damping or signal filtering without adding a lag time, and enable smarter predictive maintenance based on the long-term trend of an electrode’s deviation from the middle value. A single electrode can be manually or automatically retracted for cleaning, rejuvenation and calibration with the controller automatically. More intelligent maintenance and better measurement performance reduces lifecycle cost and increases process efficiency and capacity.
Greg: What are some good calibration practices?
pH Greg: In general, electrodes should have a two-point buffer calibration before they are commissioned and after they are cleaned or the gel layer is rejuvenated. When an electrode has been in service long enough for the reference electrode to reach equilibrium, a “grab sample” process standardization should be done.
Process standardizations should only be performed when three or more samples—preferably spread over several days—show the same relative offset to prevent the common problem of chasing calibration adjustments. Samples that come back with alternating error signs are a sure sign of mixing or measurement noise. Chasing noise will cause additional errors and create additional work.
Data analysis and selecting the middle value of three electrodes or two electrodes and one estimator can be used to improve the understanding of what noise is and what is real to reduce the frequency of process samples. In the “grab sample” method, the measured pH must be time synchronized with the time the sample was taken from the process (not when it was measured in the lab). Also, the sample must be at the same point in the process as the pH measurement electrode, and the lab measurement must be made at the same temperature or corrected to the same temperature in the process as the pH measurement electrode. All the concerns about the lab sample mixture changing because of absorption, evaporation, dissolution, and reaction discussed.
Greg: Advances in glass technology and removable specialized liquid junctions with options for automated jet washing and retraction with automated standardization and conditioning, can greatly improve life and accuracy. For more detail on how to become famous not only for the pH measurement but also the pH control system see Advanced pH Measurement and Control – Digital Twin Synergy and Advances in Technology Fourth Edition, 2024.
Top 10 signs of famous pH measurement achievements
- Operators thank you for pH improvements by meals given you in control room
- Pictures of pH electrodes hanging on the walls of plant offices
- Managers want copies of your pH book
- Process engineers want to become Instrument engineers
- Plant standardizes on middle signal selection
- Engineers show pictures of pH electrodes at parties
- Universities want you to teach courses on pH
- Neighbors want you improve their pool pH
- You are asked to do a TV documentary on pH measurement
- You are asked to appear on late night TV Talk show to tell your pH
About the Author
Greg McMillan
Columnist
Greg K. McMillan captures the wisdom of talented leaders in process control and adds his perspective based on more than 50 years of experience, cartoons by Ted Williams and Top 10 lists.